Alkdehyde C-H Cross-Coupling via Metallaphotoredox/HAT Catalysis
X. Zhang, D. W. C. MacMillan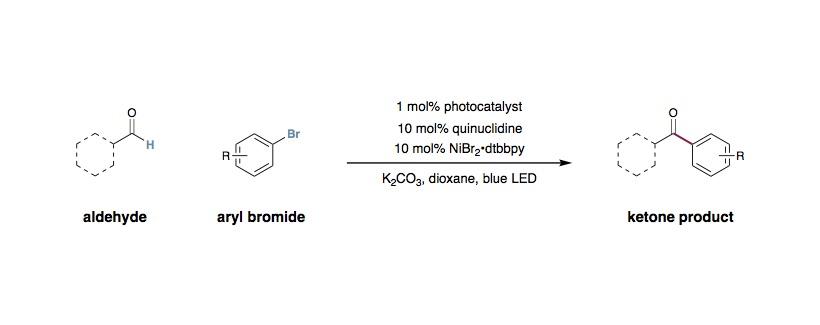
Light source:
Kessil blue LED. Use 1 light for 1 8 mL vial, and 2 lights for 1 40 mL vial.
General procedure:
For the arylation and vinylation:
To an 40 mL vial equipped with a stir bar was added photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 (4 μmol, 0.01 equiv.), aryl/vinyl halide (0.40 mmol, 1 equiv.), aldehyde (0.80 mmol, 2 equiv.), quinuclidine (40 μmol, 0.1 equiv.), and anhydrous potassium carbonate (0.60 mmol, 1.5 equiv.). The vial was sealed and placed under nitrogen before 1,4-dioxane (6 mL) was added. To a separate vial was added NiBr2·glyme (40 μmol, 0.1 equiv.) and 4,4’-di-tert-butyl-2,2’-bipyridine (40 μmol, 0.1 equiv.). The precatalyst vial was sealed, purged with nitrogen, dissolved in 1,4-dioxane (6 mL) and then sonicated until it became homogeneous. Subsequently, the precatalyst solution was syringed into the reaction vessel and the solution was degassed by sparging with nitrogen for 15 minutes before sealing with parafilm. The reaction was stirred and irradiated using 34 W blue LED lamps (6 cm away, with cooling fan to keep the reaction at room temperature) for 20 hours. The reaction mixture was removed from the light, cooled to ambient temperature, diluted with water and EtOAc, and the aqueous layer was extracted with three portions of EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, filtered, and concentrated. The residue was purified by flash chromatography on silica gel to afford the desired ketone product.
For the alkylation:
To an 40 mL vial equipped with a stir bar was added photocatalyst Ir[dF(Me)ppy]2(dtbbpy)PF6 (9 μmol, 0.02 equiv.), quinuclidine (45 μmol, 0.1 equiv.), and anhydrous sodium carbonate (1.35 mmol, 3 equiv.). The vial was sealed and placed under nitrogen before acetone (5 mL) was added. To a separate vial was added NiBr2·glyme (45 μmol, 0.1 equiv.) and 4,4’-di-tert-butyl-2,2’-bipyridine (45 μmol, 0.1 equiv.). The precatalyst vial was sealed, purged with nitrogen, dissolved in acetone (5 mL) and then sonicated until it became homogeneous. Subsequently, the precatalyst solution was syringed into the reaction vessel and the solution was degassed by sparging with nitrogen for 15 minutes. Alkyl halide (0.45 mmol, 1 equiv.) and aldehyde (1.35 mmol, 3 equiv.) were then added. The reaction vial was then sealed with parafilm, placed 6 cm away from one blue LED, and irradiated (heated to approximately 55 °C by the blue LED without fan cooling). After 20 h, the reaction mixture was purified directly by flash chromatography on silica gel to afford the desired ketone product.
Tips and tricks:
For the arylation and vinylation:
- K2CO3 is universally the most effective base for the arylation and vinylation. For a homogeneous reaction, quinuclidine (1.1~1.2 equivalents) or quinuclidine (0.1 equivalents) with 2,6-lutidine will provide the product but in a slightly diminished yield.
- Aldehyde equivalents could be played around with for problematic substrates. Sometimes, higher aldehyde loadings give higher efficiency of the reaction.
- The reaction can be concentrated to 0.1~0.2 M and still produce good yields of product.
- Nickel loading can be reduced to 2% and doesn’t dramatically impact the efficiency of the reaction.
- The purity of the aldehyde substrates can be important to achieve high yield. We suggest that aldehydes (if liquid) are distilled prior to use; aldehydes (if solid) are purified by column chromatography prior to use.
For the alkylation:
- Na2CO3 is universally the most effective base for the alkylation.
- Photocatalyst Ir[dF(Me)ppy]2(dtbbpy)PF6 are more effective than Ir[dF(CF3)ppy]2(dtbbpy)PF6 in this transformation. If starting materials are remaining, try additional quantities of photocatalyst or increasing the reaction temperature.
- Dioxane is comparable to acetone in most reactions for the alkylation.
- Aldehyde and quinuclidine equivalents could be played around with for problematic substrates.