Alkylation of sp3 C-H Bonds via Metallaphotoredox/HAT Catalysis
C. Le, Y. Liang, R. W. Evans, X. Li, D. W. C. MacMillan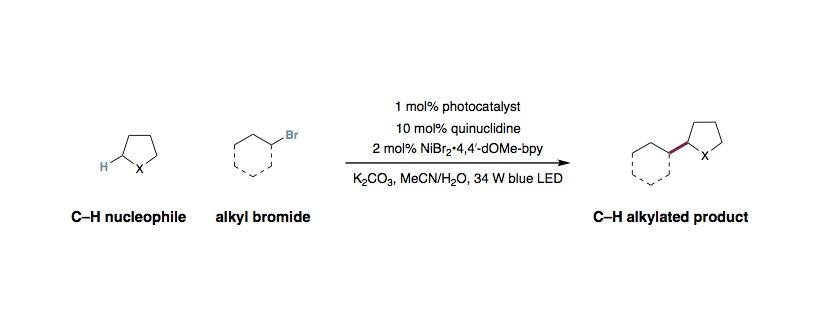
Light source:
34 W blue LED, two 8 mL vials can be placed in front of each lamp.
General procedure:
To an oven-dried 8-mL vial equipped with a stir bar was added Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (5.6 mg, 5.0 μmol, 0.01 equiv.), Ni(II) salt and bipyridyl ligand. MeCN was added and the solution was stirred under nitrogen for 15 minutes to allow for complete complexation. Quinuclidine (as a MeCN solution), inorganic base, amine (1.00 mmol, 2.0 equiv.) and alkyl halide (0.50 mmol, 1.0 equiv) were added, followed by addition of water. The reaction was sparged with nitrogen for 15 minutes at 0 ºC (ice water bath) before being parafilmed and placed 5 cm away from 34W blue LEDs without fan. The temperature of the reaction is approximately 50 °C without fans, and 35 ºC with fans. After 24 hours, the reaction was quenched via exposure to air. The organic layer was diluted with EtOAc then washed with NaHCO 3 (saturated, aq) and brine. The organic layer was then separated, dried with MgSO 4 and concentrated to give the crude product. Purification by column chromatography yields the pure product. In all reported examples, the remaining untouched nucleophile can be recovered during purification in good yields.
Tips and tricks:
- The reaction has been scaled up to 3.0 mmol without decrease in efficiency. Stirring to ensure efficient mixing of the biphasic reaction mixture is crucial for high efficiency.
- During optimization studies, we observed that the success of the reaction relies heavily on the medium of the reaction. As reported, MeCN, acetone and EtOAc were the three best solvents. In addition, equivalent of water is important and vary from substrate to substrate. For new substrates, we typically evaluate the optimal equivalent of water before moving on to different parameters.
- For many substrates, we found that maintaining the reaction temperature at 50 ºC resulted in higher yield. This can be accomplished by simply turning off the fans.
- Nickel source and ligand does not result in drastic change in yield and the reaction profile.
- For methylation, quinuclidine and water were excluded from the reaction mixture. This was the only case in which we found the addition of these components resulted in lower efficiency.
- The C–H nucleophjiles should be used in excess of the alkyl halide (typically 3:1 to 5:1). While the remaining starting material can be reisolated in good mass balance, it is important to maintain a high concentration of the nucleophile to get good efficiency. Use of excess alkyl halide often result in low yield of the desired product.
- It is worth noting that while alkylation of ether moieties is shown to be feasible, these reactions often require large excess of the nucleophile to proceed in good yields. Consistent with this observation, in cases where amide and ether motifs are both present in the reaction mixture, alkylation was only observed at the α-C–H to the nitrogen. We hypothesize that the difference in hydricity of the α-C–H, coupled with the nucleophilicity of the radical are major contributors to this selectivity.