Double Decarboxylative Oxalate Michael Addition
C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. C. MacMillan, L. E. Overman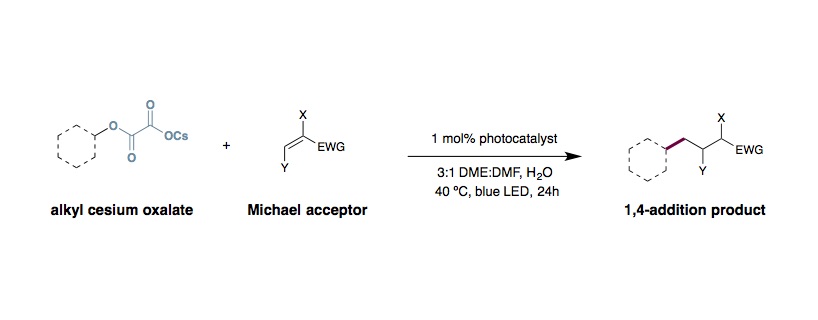
Light source:
Kessil Blue LED lamp. One lamp is sufficient for 3x8mL vials.
General procedure:
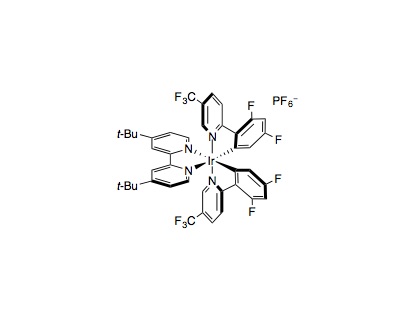
An 8 mL-scintillation vial equipped with a Teflon septum and magnetic stir bar was charged with cesium oxalate (0.55 mmol, 1.1 equiv.) and Ir[dF(CF3)ppy)]2(dtbbpy)PF6 (0.005 mmol, 1 mol%). A 3:1 mixture of DME:DMF (5 mL, 0.1 M) was added, followed by water (5.0 mmol, 10 equiv.) and ethyl acrylate (0.5 mmol, 1.0 equiv.). The reaction mixture was degassed by sparging with argon for 15 min and the vial was sealed, stirred, and irradiated for 24 h with the reaction temperature rising to 40 ºC because of heat given off from the LEDs. The reaction mixture was diluted with sat. LiCl(aq) (25 mL) and the aqueous phase was extracted with Et2O (2×25 mL). The combined ethereal extracts were dried over Na2SO4 and concentrated. The crude material was purified by flash column chromatography on silica gel.
Tips and tricks:
- Oxalate equivalents can be increased to 1.5 equiv for improved yields in some cases.
- You can hydrolyze the mixed-oxalate methyl ester in situ with CsOH with comparable efficiency.
- Lithium, sodium, and potassium oxalates can all be utilized with minimal loss in efficiency.