Energy Transfer-Mediated C-O Coupling of Carboxylic Acids with Aryl Halides
E. R. Welin, C. C. Le, D. M. Arias-Rotondo, J. K. McCusker, D. W. C. MacMillan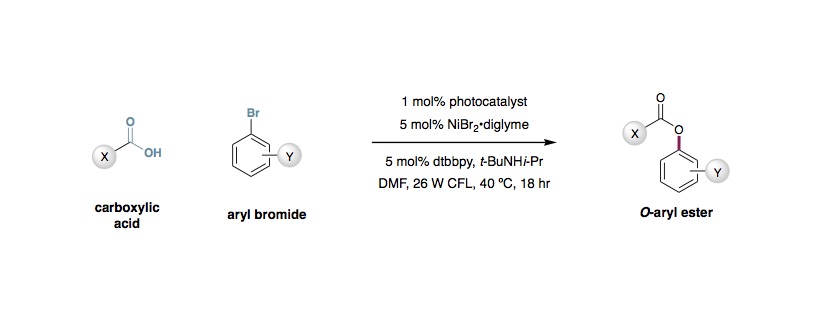
Light source:
2x 26 W CFLs, one on either side, 1–2 cm from the vial. Each setup can support 4-5 8 mL vials effectively. No cooling fan is necessary.
General procedure:
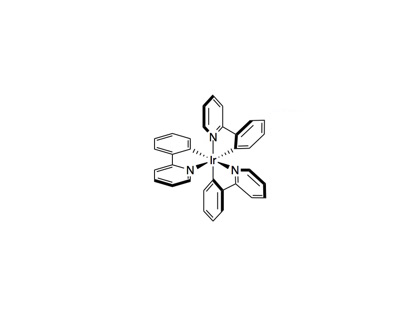
Nickel(II) Bromide•diglyme (14.1 mg, 0.04 mmol, 5 mol%), 4,4´-di-tert-butyl-2,2´-dipyridyl (10.7 mg, 0.04 mmol, 5 mol%) and tris[2-phenylpyridinato-C2,N]iridium(III) [Ir(ppy)3, 1, 5.2 mg, 8.0 µmol, 1 mol%), bromoarene (0.8 mmol, 1.0 equiv) and carboxylic acid (1.6 mmol, 2.0 equiv) were weighed into an oven-dried 8 mL vial and and dimethylformamide (4 mL) was added, providing a bright green solution. Finally, N-tert-butyl-isopropylamine (254 µL, 1.6 mmol, 2.0 equiv). While stirring, nitrogen was bubbled through the solution for 15 min, then the vial was sealed with parafilm and placed in between two 26 W compact fluorescent lights (CFL, 1-2 centimeter from either side of the vial), allowing the temperature to rise due to proximity to the lights. After 18 hr, the solution was poured into an aqueous lithium chloride (20% saturated aqueous LiCl, 80% water) and this solution was extracted with 3 portions of ethyl acetate (10 mL each). The combined organic phases were washed with aqueous lithium chloride (50% saturated aqueous LiCl, 50% water), dried over MgSO4 and concentrated. The product was purified by flash column chromatography.
Tips and tricks:
1. Use of diisopropylamine in place of N–tert-butylisopropylamine provides nearly equal yields, however the acid-base salts it forms were typically quite insoluble. In our hands, this did not affect the outcome of the reaction.
2. Aryl bromides provided higher reactivity than aryl iodides. Electron-deficient bromides are the ideal reaction partners.
3. All carboxylic acids that we tested provided aryl ester products. Acids that also bear free hydroxyl groups were found to inhibit catalysis somewhat, but still provided 40–50% yield.
4. The only impurities we observed were remaining aryl halide and the phenol corresponding to the aryl halide used. The former is easily removed by column chromatography and is a sign that a longer reaction time is required. The latter can be removed by washing with saturated aqueous K2CO3 prior to drying over MgSO4 and is an indication that a shorter reaction time would be useful.
5. Some of the products crashed out of EtOAc/hexanes during chromatography. Using 1:1 EtOAc:DCM in place of straight EtOAc behaves identically and allows full solubility.
6. The best results were obtained with a 2:1 acid/base:aryl bromide ratio. Lowering this ratio causes lower yields and necessitates longer reaction times.