Alkylation of Heterocycles Using Simple Alcohols
J. Jin, D. W. C. MacMillan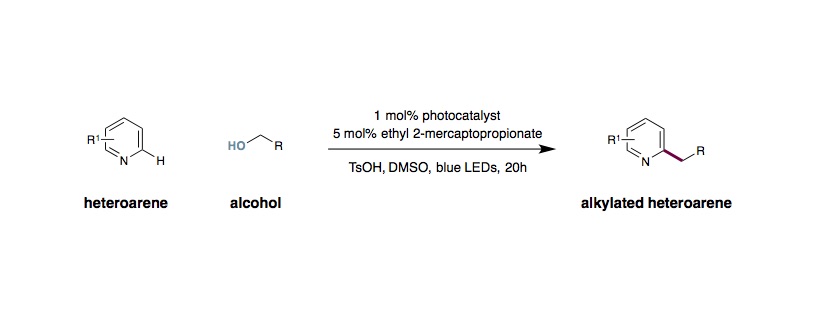
Light source:
Three double density sapphire Blue LED strips.
General procedure:
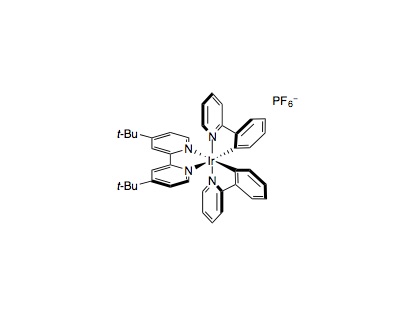
To an 8 mL vial equipped with a Teflon septum and a magnetic stir bar was charged Ir(ppy)2(dtbbpy)PF6 (0.005 mmol, 1 mol%), TsOH (1.0 mmol, 2.0 equiv.), heteroarene (0.50 mmol, 1.0 equiv.), alcohol (5.0 mmol, 10.0 equiv.) and DMSO (2.0 mL, 0.25 M). The reaction mixture was degassed by sparging with nitrogen for 10 min and then methyl thioglycolate (0.025 mmol, 5 mol%) was added. The reaction then placed approximately 2 cm away from the light source and irradiated and stirred (300-500 rpm) at room temperature under a mini fan. Upon reaction completion as judged by TLC and LCMS (20–48 hours), the reaction mixture was diluted with 1 M NaOH aqueous solution (2 mL) and CH2Cl2 (20 mL), washed with brine (3×10 mL), dried over Na2SO4, and concentrated in vacuo. Purification of the crude product by flash chromatography on silica gel afforded the desired product.
Tips and tricks:
- For heteroarene methylation, using a large excess of MeOH is beneficial. For a reaction in 2 mL of DMSO, 2 mL of MeOH was added instead of the normal 10 equiv. alcohol.
- Diluting the reaction further can often be beneficial.
- Increasing the amount of TsOH (3 equiv.) or thiol catalyst (10 or 20 mol%) can often help with difficult substrates.
- Increasing alcohol equivalents is always beneficial. Decreasing alcohol equivalents (as low as 2–5 equiv.) is typically fine, but the reaction time must be increased.
- If you want to replace DMSO as solvent, MeCN is the best alternative.
If you want to replace TsOH as acid, concentrated H2SO4 is the best alternative.