Metallaphotoredox C–N Coupling of Amines with Aryl Halides
E. B. Corcoran, M. T. Pirnot, S. Lin, S. D. Dreher, D. A. DiRocco, I. W. Davies, S. L. Buchwald, D. W. C. MacMillan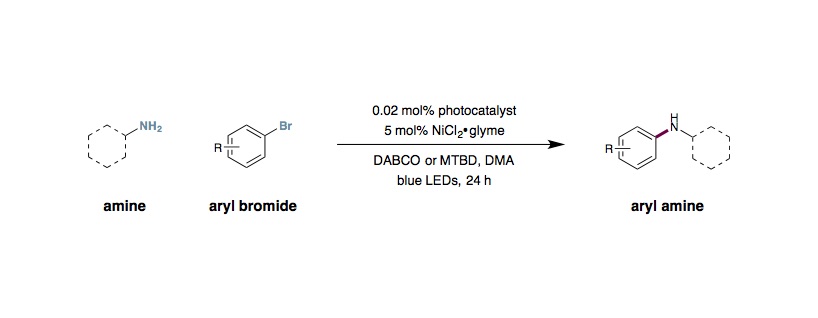
Light source:
Kessil Blue LED lamp. One lamp is sufficient for 3x8mL vials.
General procedure:
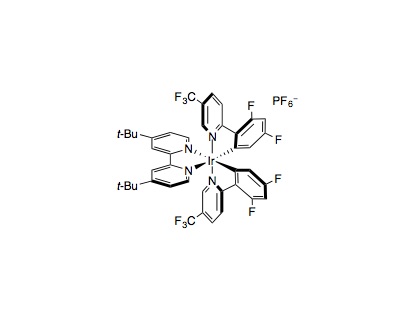
To an oven-dried 8 mL vial equipped with a magnetic stir bar under ambient atmosphere, aryl halide (0.25 mmol, 1.0 equiv.), amine (0.375 mmol, 1.5 equiv.), DABCO (0.45 mmol, 1.8 equiv.), and Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.005 mmol, 0.02 mol%) were added, followed by DMA (0.5 mL). Then, NiBr2•DME or NiCl2•DME (.0125 mmol, 5 mol%) was added as a solution in DMA (0.5 mL), which had been sonicated to enhance solubility. This brings the total volume to 1.0 mL DMA (0.25 M). The vial was then placed under an atmosphere of nitrogen, cooled to –78 °C, and degassed via vacuum evacuation (5 min), backfilled with nitrogen, and warmed to ambient temperature. This sequence was repeated three times, then the vial was sealed with parafilm. The vial was placed 6 cm away from one 34 W blue LED lamp, with the LED shining directly at the side of the vial. The reaction was either maintained at ambient temperature (approximately 25 °C) by fan cooling (one fan per LED, 6 cm away from LED) or allowed to warm to approximately 55 °C by irradiation with the LED in the absence of fan cooling. The reaction was stirred at approximately 500 rpm for several hours (reactions time vary with substrate), and then the LED lamp was turned off. The product was directly purified by flash column chromatography on silica gel.
Tips and tricks:
- For cases in which the coupling amine is not sufficiently nucleophilic (g., fluorinated amines, aniline and N-Boc piperazine), the reaction proceeds more efficiently at 55 °C (i.e., without fan cooling).
- For electron-neutral or electron-rich aryl halides, the optimal reactivity is observed at 55 °C (e., without fan cooling). Additionally, lowering the photocatalyst loading to 0.005 mol% or 0.0002 mol% is often beneficial, as is the use of DMSO in lieu of DMA as solvent and the use of MTBD in lieu of DABCO as base.
- If a substantial amount of protodehalogenated arene side product is observed, try lowering the photocatalyst loading.
- If the reaction proceeds slowly at elevated temperatures, try lowering the photocatalyst loading. If that is unsuccessful, try adjusting the nickel catalyst loading to 1 mol% or 10 mol%.
- The bases which perform the best in these reactions are: DABCO, MTBD, and quinuclidine. The use of inorganic bases was found to be deleterious.
- For employing the HCl salts of amines directly in the cross-coupling, use 2.0 equivalents of MTBD as base and heat the reaction to 55 °C (e., without fan cooling).
- High-intensity blue LED strips can also be used as the light source, although longer reaction times and higher photocatalyst loadings (approximately 1 mol%) are required.
- Aryl iodides and electron-deficient aryl chlorides are also competent coupling partners in this amination protocol.
For ease of reaction set up, a solution of photocatalyst in DMA or DMSO can be used. The solution can be stored under ambient atmosphere for several months with no decreased reactivity being observed.