α-Alkylation of Alcohols via HAT
J. L. Jeffrey, J. A. Terrett, D. W. C. MacMillan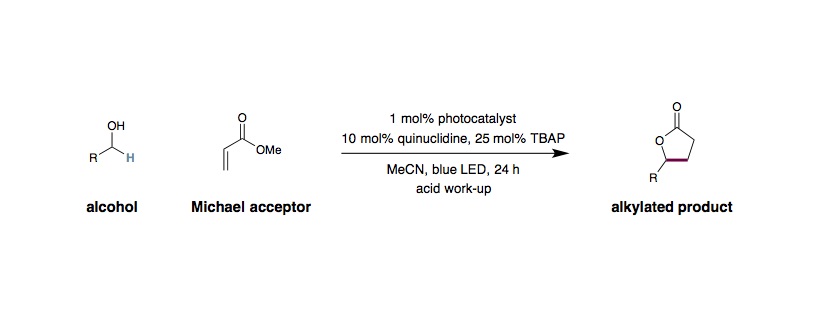
Light source:
Kessil Blue LED lamp.
General procedure:
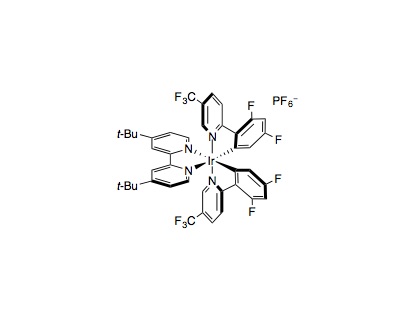
Alkylation/lactonization: An 8 mL glass vial equipped with a Teflon septum and magnetic stir bar was charged with Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.0025 mmol, 1 mol%), quinuclidine (0.025 mmol, 10 mol%), and tetra-N-butylammonium phosphate (0.0625 mmol, 25 mol%), followed by CH3CN (0.31 mL, 0.8 M), 1-hexanol (0.50 mmol, 2.0 equiv.) and methyl acrylate (0.25 mmol, 1.0 equiv.). The resulting solution was then sparged with N2 for 3 minutes. The vial was sealed and placed approximately 3 inches away from a Kessil® LED lamp. The reaction mixture was stirred and irradiated for 24 h. The internal temperature was maintained at approximately 27 °C by a fan placed approximately 6–10 inches above the vial. Upon completion, Amberlyst® 15 (dry, 400 mg/mmol acrylate) was added to the reaction mixture in one portion. The resulting mixture was heated with stirring at 50 °C for 3 h. After cooling to room temperature, the Amberlyst® 15 beads were removed by filtration and the reaction mixture was concentrated in vacuo. Purification of the crude product by flash column chromatography on silica gel afforded the desired product.
Alkylation: An 8 mL glass vial equipped with a Teflon septum and magnetic stir bar was charged with Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.0025 mmol, 1 mol%), quinuclidine (0.025 mmol, 10 mol%), and tetra-N-butylammonium phosphate (0.0625 mmol, 25 mol%), followed by CH3CN (0.31 mL, 0.8 M), an alcohol (0.50 mmol, 2.0 equiv.) and a Michael acceptor (0.25 mmol, 1.0 equiv.). The resulting solution was then sparged with N2 for 3 minutes. The vial was sealed, placed approximately 3 inches away from a Kessil® LED lamp, and stirred and irradiated for 24 h with fan cooling as described above. Upon completion, the reaction mixture was concentrated in vacuo. Purification of the crude product by flash column chromatography on silica gel afforded the desired product.
Tips and tricks:
- Some suitable solvents for most substrates, in order of best to worst, are: CH3CN > DME >DMF.
- The reaction works best (highest efficiency and fastest rate) when either the alcohol or the Michael acceptor is present in excess (2 equiv. in most cases).
- If a given alcohol substrate does not provide a useful yield of product, try increasing the loading of the Michael acceptor.
- If a given Michael acceptor does not provide useful levels of efficiency, try increasing the loading of the alcohol.
- If the yield does not improve, it may be necessary to extend the reaction time beyond the typical 24 h.
- Double density sapphire blue LED strips may be used as the light source. However, longer reaction times are typically required with blue LED strips.
- For the lactonization, trifluoroacetic acid (3 equiv.) may be used in place of Amberlyst® 15 beads. However, the use of Amberlyst® 15 beads facilitates purification of the products in many cases.