Decarboxylative Michael Addition
L. Chu, C. Ohta, Z. Zuo, D. W. C. MacMillan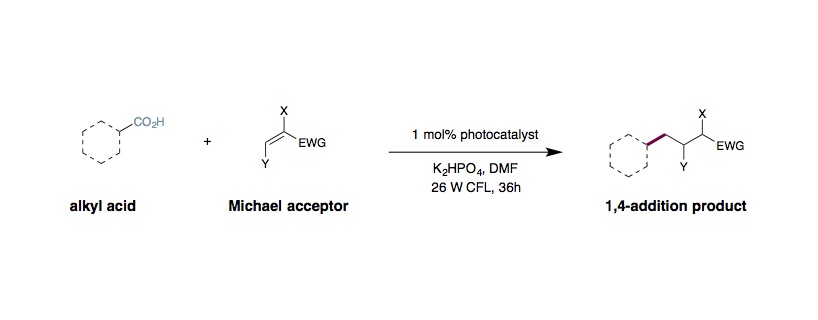
Light source:
26W CFL lamp. One work lamp is sufficient for as many vials as fit along its length.
General procedure:
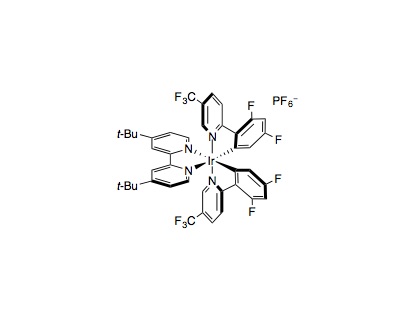
An oven-dried 8 mL vial equipped with a Teflon septum and magnetic stir bar was charged with Ir[dF(CF3)ppy]]2(dtbbpy)PF6 (0.002 mmol, 1 mol%), carboxylic acid (0.2 mmol, 1.0 equiv.), K2HPO4 (0.24 mmol, 1.2 equiv.), Michael acceptor (0.2 mmol, 1.0 equiv.), and DMF (0.5 mL, 0.4 M), in that order. The reaction mixture was degassed with nitrogen for 15 min, then stirred (~800 rpm) and irradiated with a 26 W fluorescent lamp (at approximately 2 cm away from the light source), with no fans necessary for cooling. After 36h, the reaction mixture was diluted with saturated aqueous NaHCO3 solution, extracted with Et2O (3 × 50 mL). The combined organic extracts were washed with water and brine, dried over MgSO4 and concentrated in vacuo. Purification of the crude product by flash chromatography on silica gel afforded the desired product.
Tips and tricks:
- If the Michael acceptor is volatile, add it after degassing.
- For acids that are not providing optimal yields, consider changing the base, solvent, or concentration (in that order).
- For acceptors that are not providing optimal yields, consider changing solvent or base (in that order).
- DMA is the second best solvent. Li2CO3 and CsOAc are the best alternative bases.
- If the Michael acceptor is prone to polymerization, simply increase the equivalents.