Metallaphotoredox C–O Coupling of Alcohols with Aryl Halides
J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff, D. W. C. MacMillan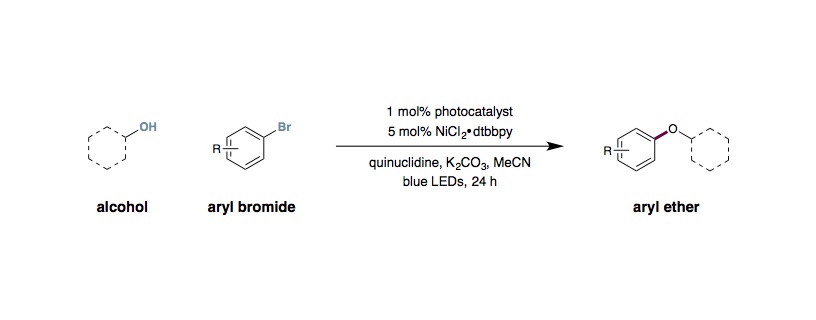
Light source:
Three double density sapphire blue LED strips.
General procedure:
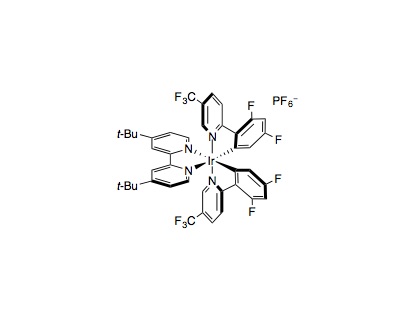
To an 8 mL vial containing a solution of the arene (1.00 mmol, 1.0 equiv.), quinuclidine (0.100 mmol, 10 mol%), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.010 mmol,1 mol%) and potassium carbonate (1.00 mmol, 1.0 equiv.) in acetonitrile (2.0 mL) was added a solution of NiCl2⋅DME (0.050 mmol, 5 mol%) and 4,4′-di-tert-butyl-2,2′-dipyridyl (0.05 mmol, 5 mol%) in acetonitrile (2.0 mL), bringing the total volume to 4 mL MeCN (0.25 M). The vial was placed under an atmosphere of nitrogen, then the alcohol (1.00–3.00 mmol, 1.0–3.0 equiv.) was added. The reaction mixture was then cooled to –78 °C and degassed via vacuum evacuation (5 min), backfilled with nitrogen, and then warmed to room temperature. This process was repeated three times, then the vial was sealed with parafilm, placed 1 cm away from three blue LED strips, and irradiated with blue LEDs under fan cooling (to maintain at room temperature). After 24 hours, the reaction mixture was diluted with ethyl acetate (10 mL) then poured into a separatory funnel containing water (10 mL). The aqueous phase was separated and extracted with ethyl acetate (3×10 mL). The combined organic extracts were washed with brine (10 mL), dried (Na2SO4), and then concentrated in vacuo. Purification of the crude material by flash column chromatography on silica gel using the indicated solvent system afforded the desired aryl ether product.
Tips and tricks:
- A compact fluorescent lightbulb (CFL) can be used in place of blue LED strips for comparable reaction efficiency.
- For good reaction efficiency, an excess of the alcohol is typically used (1.5 equiv. for primary alcohols and 3 equiv. for secondary alcohols). The stoichiometry can be inversed with the aryl halide in excess, resulting in comparable levels of efficiency.
- MeCN is the optimal solvent for the transformation; however, acetone and ethyl acetate work comparably.
- For some heteroaryl halides, such as pyrimidines, the reaction can be slow. The reaction rate can be improved upon heating the reactions to 45–50 °C (accomplished simply by removal of the fan cooling).
- While the reaction works best when aryl bromides are employed, aryl iodides can be used instead by switching quinuclidine for DBU as the sacrificial reductant.
- To accomplish C–O coupling with H2O as the nucleophile (thereby forming phenol products), a solvent switch is important. While the reaction efficiency is low in MeCN, this can be dramatically improved by using 2-methyl THF as the solvent.
- Under the optimized conditions, the C–O coupling reaction is heterogeneous. If a homogeneous reaction is required, the K2CO3 can be replaced with 1 equiv. of quinuclidine as the base, resulting in identical reaction efficiency.