Metallaphotoredox Decarboxylative Vinylation
A. Noble, S. J. McCarver, D. W. C. MacMillan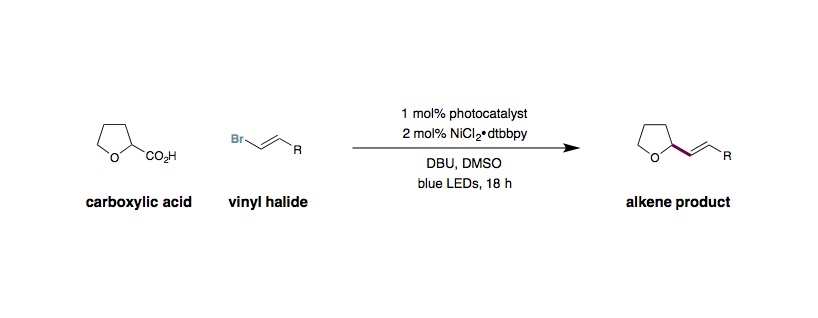
Light source:
Kessil Blue LED lamp. One lamp can be utilized for up to 3x8mL vials.
General procedure:
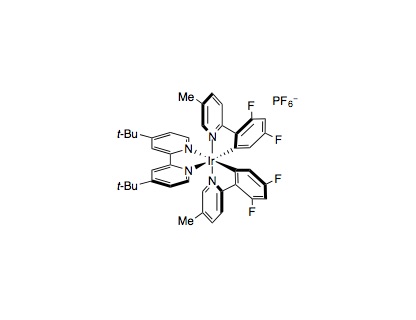
To an oven dried 8 mL vial equipped with a cross-shaped stir bar was added Ir[dF(Me)ppy]2(dtbbpy)PF6(0.005 mmol, 1 mol%), the carboxylic acid (if solid) (0.80 mmol, 1.6 equiv.), and the vinyl halide if solid (0.50 mmol, 1.0 equiv.). The vial was sealed and evacuated then backfilled with nitrogen three times. DMSO (4.0 mL, 0.125 M) was added to the vial, followed by the carboxylic acid (if liquid), 1,8-diazabicycloundec-7-ene (DBU) (0.80 mmol, 1.6 equiv.), and the vinyl halide (if liquid). NiCl2⋅glyme (0.01 mmol, 2 mol%) and dtbbpy (0.01 mmol, 2 mol%) were added as a solution in DMSO (1.0 mL, 0.01 M). The reaction mixture was degassed by sparging with nitrogen while stirring for 15 min before sealing the vial with Parafilm. The reaction was stirred at 800 rpm and irradiated with a 34 W blue LED lamp (placed 4 cm away), with a single fan placed overhead for cooling, until complete consumption of the vinyl halide. The reaction was diluted with water (15 mL) and brine (5 mL) and the product extracted into Et2O (3 x 20 mL). The combined organic extracts were combined and washed with water (2 x 15 mL), brine (10 mL), dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography yielded the vinylated product.
Tips and tricks:
- It is critical that the nickel catalyst and ligand are premixed as a stock solution and sonicated until homogeneous. Prepared stock solutions can be used for up to a week if stored under N2 in a scintillation vial. The catalyst stock solution is always added to the reaction mixture last, immediately before sparging.
- Performing reactions is DMSO at 0.1 M with DBU as a base is effective for most substrates. However, there are some cases where other conditions lead to higher product yields:
- For NHBoc amino acids, DMA at 0.1 M and Cs2CO3 should be utilized.
- For sterically hindered vinyl halides and acyclic a–oxy carboxylic acids, the vinyl bromide should be used in place of the vinyl iodide. Additionally, the reactions should be run in DMA at 0.1 M using Cs2CO3 as a base and 0.06 equiv of N-Boc-benzylamine added.
- For carboxylic acids lacking a heteroatom and tetrahydropyran carboxylic acids, reactions should be performed in DMSO at 0.025 M with Cs2CO3 as the base.