Metallaphotoredox Decarboxylative Acylation
L. Chu, J. M. Lipshultz, D. W. C. MacMillan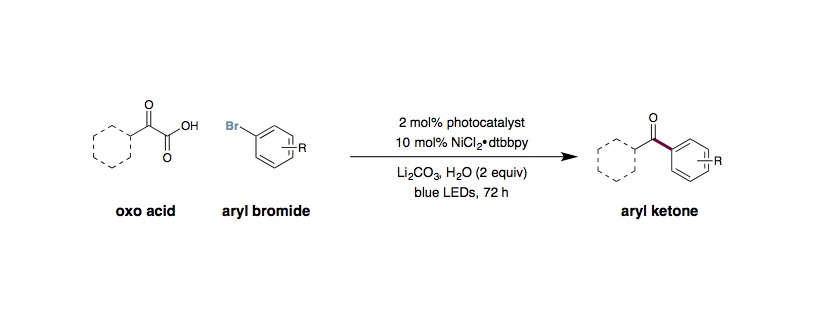
Light source:
Kessil Blue LED lamp. One lamp can be utilized for 2x40mL vials, or 4x8mL vials.
General procedure:
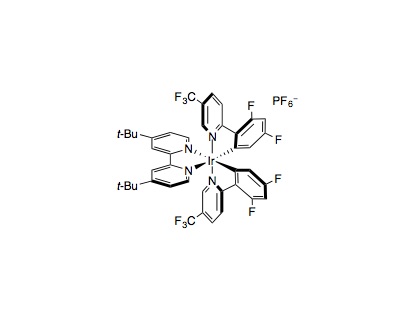
An oven-dried 40 mL vial equipped with a Teflon septum cap and magnetic cross-shaped stir bar was charged with Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.01 mmol, 2 mol%), NiCl2⋅glyme (0.05 mmol, 10 mol%), 4,4¢-di-tert-butyl-2,2¢-bipyridyl (0.075 mmol, 15 mol%), the keto acid (1.0 mmol, 2.0 equiv.), aryl halide (0.5 mmol, 1.0 equiv.), and Li2CO3 (1.0 mmol, 2.0 equiv.) from a bottle stored in a desiccator. To this vial was added DMF (25 mL, 0.02 M, from our solvent system or a Sure/Sealâ bottle) and water (1.0 mmol, 2.0 equiv.). The reaction mixture was degassed for 30 minutes with either argon or nitrogen stream then sealed with a very large amount of parafilm. If one of the coupling partners was volatile, that was simply added by syringe after the degassing. The vial was irradiated with 34W Blue LED lamp (8 cm away from the vials) and stirred (1000 rpm) for 72 hours, with cooling from a fan. After 72 hours, the reaction was diluted with H2O (25 mL) and extracted with Et2O (3×75 mL). The combined organic layers were washed with H2O (3×25 mL), dried over MgSO4, filtered, and concentrated in vacuo. Purification of the crude product by flash chromatography on silica gel provided the acylated product.
Tips and tricks:
- Numerous ketoacids (such as the aliphatic and mesityl ketoacids) proceed much quicker than 72 hours. They are mostly done in 24–36 hours. However, occasionally longer reaction times are needed- this is probably where you should start if you see any acid or halide remaining.
- Acid equivalents could be played around with for problematic substrates. Sometimes, higher acid loadings give higher conversion of the aryl halide to the acylated product. For efficient substrates, acid loading can be dropped to 1.2–1.5 equiv.
- The ligand has very little effect on the efficiency- dtbbpy and bpy are almost equivalent, and there doesn’t seem to be any improvement with other ligands.
- You can change the stoichiometry of acid/halide and still get reasonable yield, but recognize that a sacrificial amount of acid must be oxidized to generate the Ni(0) complex.
- DMSO and DMA are the best alternative solvents, but DMF is generally the best by a reasonable amount.
- The reaction is not particularly sensitive to heat. The vials reached around 37 °C with more efficient cooling having no effect on the reaction.