Metallaphotoredox Decarboxylative Arylation
Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle, D. W. C. MacMillan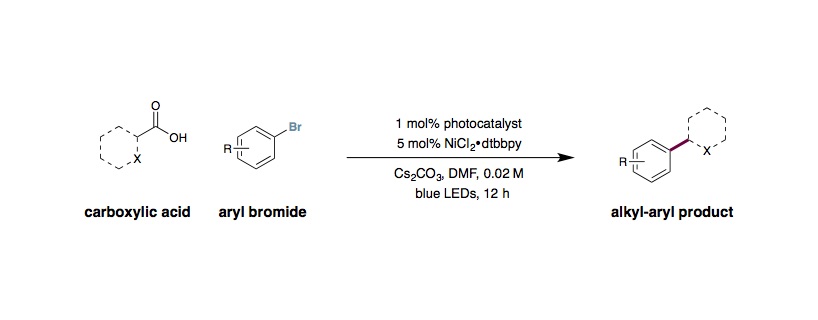
Light source:
Kessil Blue LED lamps. For 40 mL vials, use 2 lights per reaction. For 8 mL vials, use 1 light per reaction.
General procedure:
For α-oxy or amino carboxylic acids:
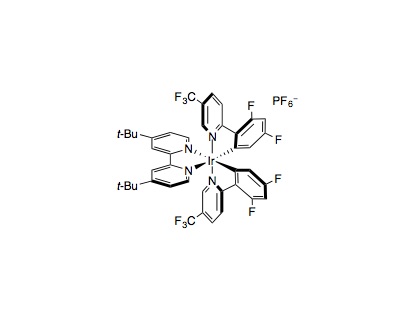
To a 8 mL vial equipped with magnetic stir bar, Ir[dF(CF3)ppy]2(dtbbpy)PF6 (0.005 mmol, 1 mol%), NiCl2⋅glyme (0.025 mmol, 5 mol%), and dtbbpy (0.025 mmol, 5 mol%) were added. Then carboxylic acid was added (if solid) (0.75 mmol, 1.5 equiv.), followed by aryl halide (if solid) (0.50 mmol, 1 equiv.), and base (0.75 mmol, 1.5 equiv., if Cs2CO3). The vial was then placed under nitrogen dry DMA (5 mL, 0.1 M) was added, as well as the aryl halide (if liquid), carboxylic acid (if liquid), and base (0.75 mmol, 1.5 equiv., if DBU). The solution was then degassed for 15 mins by sparging with nitrogen. The vial was then sealed and the cap was wrapped in parafilm. The vial was placed approximately 8 cm away from a 34 W Blue LED, with the LED shining directly at the side of the vial. A fan was placed 6 cm above the vial and turned on to cool the vial. The reaction was stirred for 24 hours, at 700 rpm, and then the LED was turned off. The vial was poured into a mixture of water and ethyl acetate. The water layer was extracted 3 times with ethyl acetate, and then the organic layer was washed with water, dried with Na2SO4, and concentrated. The product was then purified by column chromatography.
For aliphatic carboxylic acids:
To a 40 mL vial equipped with magnetic stir bar, Ir[dF(Me)ppy]2(dtbbpy)PF6 (0.005 mmol, 1 mol%), NiCl2⋅glyme (0.025 mmol, 5 mol%), and dtbbpy (0.025 mmol, 5 mol%) were added. Then the carboxylic acid was added if solid (0.75 mmol, 1.5 equiv.), followed by the aryl halide (if solid) (0.5 mmol, 1 equiv.), and Barton’s Base (1.5 mmol, 1.5 equiv.). The vial was then placed under nitrogen and dry DMSO (5 mL, 0.1 M) was added, as well as the aryl halide (if liquid) and carboxylic acid (if liquid). The solution was then degassed for 15 mins by sparging with nitrogen. The vial was then sealed and the cap was wrapped in parafilm. The vial was placed approximately 8 cm away from 1 34 W Blue LEDs, with the LEDs shining directly at the side of the vial. A fan was placed 6 cm above the vial and turned on to cool the vial. The reaction was stirred for 24 hours, and then the LED was turned off. The vial was poured into a mixture of brine and ethyl acetate. The water layer was extracted 3 times with ethyl acetate, and then the organic layer was washed with brine, dried with Na2SO4, and concentrated. The product was then purified by column chromatography.
Tips and tricks:
- Base selection is dependent upon the aryl halide used. Unfortunately, these guidelines only apply to α-oxy or amino acids, as alkyl carboxylic acids require Barton’s Base for best results.
- For electron rich or electron neutral arenes, the iodide works best. For electron deficient and hetero aromatics, the bromide works comparably well. For 2-halopyridines, the chloride can sometimes work, especially if there is substitution at the 6-postion.
- Solvent is the most important factor. Generally, DMA, DMF, DMSO, and MeCN give the best results, but DMSO typically gives the best results for alkyl acids.
- If large amounts of dehalogenation products are formed, try lowering the nickel loading to 2.5 mol% or 1 mol%. Conversely, if large amounts of starting material are still present, try increasing the nickel loading to 7.5 mol% or 10 mol%.
- Diluting the reaction to 0.02 M can sometimes also be beneficial, as this typically reduces the amount of dehalogenated side products that are formed.
- If your aryl halide is the valuable coupling partner, increasing acid (and base) equivalents can often be beneficial.
- If a particular acid is a problem, try switching to Ir[dF(Me)ppy]2(dtbbpy)PF6 or Ir[dF(F)ppy]2(dtbbpy)PF6. These catalysts are more stable under the reaction conditions, and are necessary for any non-α-heteroatom substituted carboxylic acids. This is due to a Minisci-pathway where the generated radical deactivates the photocatalyst.
